Clinical Trial Supply and Logistics Market Size to Exceed USD 8.45 Billion by 2034
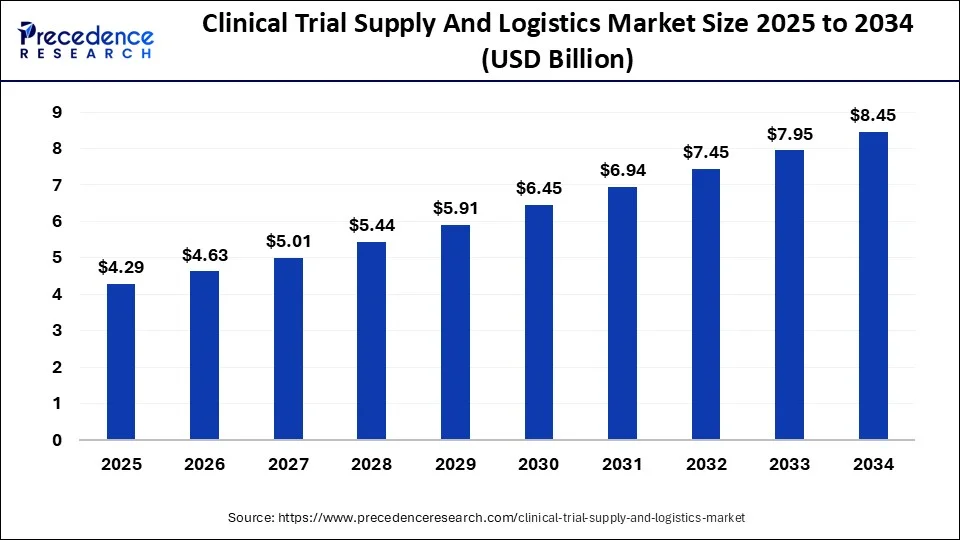
According to Precedence Research, the global clinical trial supply and logistics market size will grow from USD 4.29 billion in 2025 to nearly USD 8.45 billion by 2034, growing at a solid CAGR of 7.84% between 2025 and 2034.
Ottawa, Sept. 02, 2025 (GLOBE NEWSWIRE) -- The global clinical trial supply and logistics market size was valued at USD 3.98 billion in 2024 and is expected to exceed USD 8.45 billion by 2034. In terms of CAGR, the market is accelerating at a strong compound annual growth rate (CAGR) of 7.84% from 2025 to 2034. The clinical trial supply and logistics market grows through online platforms, digital supply chains, IoT monitoring, and patient-centric delivery.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/3303
Clinical Trial Supply and Logistics Market Highlights:
- In terms of revenue, the clinical trial supply and logistics market accounted for USD 3,980 million in 2024.
- It is projected to achieve USD 8,450 million by 2034.
- The market is expected to grow at a solid CAGR of 7.84% from 2025 to 2034.
- North America held the major market share of 36% in 2024.
- By service, the logistics & distribution segment captured the biggest market share of 27.85% in 2024.
- By service, the manufacturing segment is projected to grow at a significant CAGR from 2025 through 2034.
- By phase, Phase 3 segment contributed the highest market share of 40.41% in 2024.
- By phase, Phase 2 segment is growing at a fastest CAGR between 2025 and 2034.
- By therapeutic area, the cardiovascular diseases segment captured the highest market share of 32.33% in 2024
- By therapeutic area, the rare diseases and orphan indications segment is poised to grow at a notable CAGR from 2025 to 2034.
- By end user, the pharmaceuticals segment held the largest market share of 42.22% in 2024
Clinical Trial Supply and Logistics Market Size by Service, 2022-2024 (USD Million)
| Service | 2022 | 2023 | 2024 |
| Logistics & Distribution | 952.1 | 1,026.0 | 1,108.5 |
| Storage & Retention | 476.6 | 510.3 | 547.7 |
| Packaging, Labeling, and Blinding | 605.9 | 645.2 | 688.6 |
| Manufacturing | 767.8 | 824.6 | 887.9 |
| Comparator Sourcing | 383.4 | 409.4 | 438.1 |
| Others | 273.4 | 290.6 | 309.6 |
Clinical Trial Supply and Logistics Market Size by Phase, 2022-2024 (USD Million)
| Phase | 2022 | 2023 | 2024 |
| Phase I | 440.7 | 475.9 | 515.1 |
| Phase II | 726.0 | 776.7 | 832.9 |
| Phase III | 1,396.1 | 1,496.6 | 1,608.5 |
| Phase IV | 896.3 | 956.8 | 1,023.9 |
Clinical Trial Supply and Logistics Market Size by End User, 2022-2024 (USD Million)
| End User | 2022 | 2023 | 2024 |
| Pharmaceuticals | 1,453.3 | 1,560.9 | 1,680.7 |
| Biologicals | 1,218.3 | 1,306.1 | 1,403.7 |
| Medical Device | 787.5 | 839.0 | 896.0 |
Clinical Trial Supply and Logistics Market Size by Therapeutic Area, 2022-2024 (USD Million)
| Therapeutic Area | 2022 | 2023 | 2024 |
| Oncology | 919.1 | 987.2 | 1,063.0 |
| Cardiovascular Diseases | 1,119.4 | 1,198.7 | 1,286.8 |
| Respiratory Diseases | 773.5 | 829.9 | 892.6 |
| CNS and Mental Disorders | 647.1 | 690.2 | 738.0 |
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/3303
What is a Clinical Trial Supply and Logistics?
The clinical trial supply and logistics market is important in enabling the successful execution of clinical research in this respect by handling the end-to-end supply chain of investigational drugs, medical devices, and trial-related products. It involves proper planning, procurement, packaging, storage, distribution, and tracking to ascertain that clinical trials are conducted in a seamless fashion and satisfy high regulatory standards.
The market is experiencing strong growth due to increasing clinical trials in the world, which are supported by increasing chronic and rare diseases, personalized medicine, and innovations in biotechnology. The growing use of decentralized and virtual clinical trials is driving the urgency towards the implementation of digital supply chains and direct-to-patient delivery paradigms.
Top Trends in the Clinical Trial Supply and Logistics Market
-
Rise of Decentralized and Virtual Trials
- Clinical trials are increasingly adopting decentralized models, requiring flexible and direct-to-patient (DTP) logistics, including home delivery of investigational products and remote monitoring support.
- Clinical trials are increasingly adopting decentralized models, requiring flexible and direct-to-patient (DTP) logistics, including home delivery of investigational products and remote monitoring support.
-
Increased Demand for Cold Chain Logistics
- The growing number of biologics, gene therapies, and vaccines in trials is driving demand for ultra-cold storage and transport, with strict temperature monitoring and validated cold chain packaging.
- The growing number of biologics, gene therapies, and vaccines in trials is driving demand for ultra-cold storage and transport, with strict temperature monitoring and validated cold chain packaging.
-
Adoption of Advanced Tracking & Visibility Technologies
- Real-time shipment tracking, IoT sensors, GPS-enabled containers, and blockchain are enhancing supply chain transparency, traceability, and risk mitigation during clinical trials.
- Real-time shipment tracking, IoT sensors, GPS-enabled containers, and blockchain are enhancing supply chain transparency, traceability, and risk mitigation during clinical trials.
-
Growing Complexity of Global Trials
- With more trials spanning multiple countries and regulatory jurisdictions, there's a rising need for specialized logistics providers familiar with cross-border compliance, customs clearance, and local site coordination.
- With more trials spanning multiple countries and regulatory jurisdictions, there's a rising need for specialized logistics providers familiar with cross-border compliance, customs clearance, and local site coordination.
-
Personalized Medicine & Smaller Batch Sizes
- The rise in personalized therapies and precision medicine is leading to smaller, more complex batch production and just-in-time delivery models to avoid drug waste and improve trial efficiency.
- The rise in personalized therapies and precision medicine is leading to smaller, more complex batch production and just-in-time delivery models to avoid drug waste and improve trial efficiency.
-
Sustainability in Clinical Supply Chains
- Sponsors are looking to reduce environmental impact by adopting eco-friendly packaging, optimizing transport routes, and using recyclable temperature-control materials in trial supply logistics.
- Sponsors are looking to reduce environmental impact by adopting eco-friendly packaging, optimizing transport routes, and using recyclable temperature-control materials in trial supply logistics.
-
Increased Focus on Risk-Based Supply Planning
- Companies are using AI and predictive analytics to anticipate site demand, avoid shortages, and optimize inventory, especially in adaptive trial designs and global supply chains.
➤ Get the Full Report @ https://www.precedenceresearch.com/clinical-trial-supply-and-logistics-market
Clinical Trial Supply and Logistics Market Opportunity
Growing Partnerships Among Key Players:
The most promising opportunity in the clinical trial supply and logistics market is the growing partnerships among key players in the market. The strategic alliances are also helping businesses to increase their presence in the global marketplace, increase services, and reach more patients. These collaborative models automate the processes of the trials but also make trials more accessible to a variety of populations, including remotely isolated or underserved ones.
In addition, continuous initiatives to increase the scope of these partnerships to new territories are likely to fast-track the uptake of innovative logistics solutions across the globe.
Clinical Trial Supply and Logistics Market Major Challenge
Strict Regulatory Compliance:
Although the clinical trial supply and logistics market has a high growth potential, there is a big challenge of going through strict regulatory demands. The pharmaceutical and clinical trial industries have very strict regulatory structures that are set forth by regulatory authorities like the U.S Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
To make sure that good clinical practices (GCP) and good distribution practices (GDP) are observed, it is vital to document, exercise tight quality control, and conduct persistent audits. Such a regulatory burden hampers the execution of the trials also acts as a limiting factor to the expansion of the market, reducing scalability and operational effectiveness by the logistics providers.
Clinical Trial Supply and Logistics Market Report Scope
| Report Attributes | Statistics | |
| Market Size in 2024 | USD 3.98 Billion | |
| Market Size in 2025 | USD 4.29 Billion | |
| Market Size in 2031 | USD 6.94 Billion | |
| Market Size by 2034 | USD 8.45 Billion | |
| CAGR from 2025 to 2034 | 7.84% | |
| Largest Market | North America | |
| Base Year | 2024 | |
| Forecast Period | 2025 to 2034 | |
| Segments Covered | Service, Phase, End-user, and Therapeutic Area | |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa | |
➡️ Become a valued research partner with us ☎ https://www.precedenceresearch.com/schedule-meeting
Clinical Trial Supply and Logistics Market Key Regional Analysis
How Big is the U.S. Clinical Trial Supply and Logistics Market?
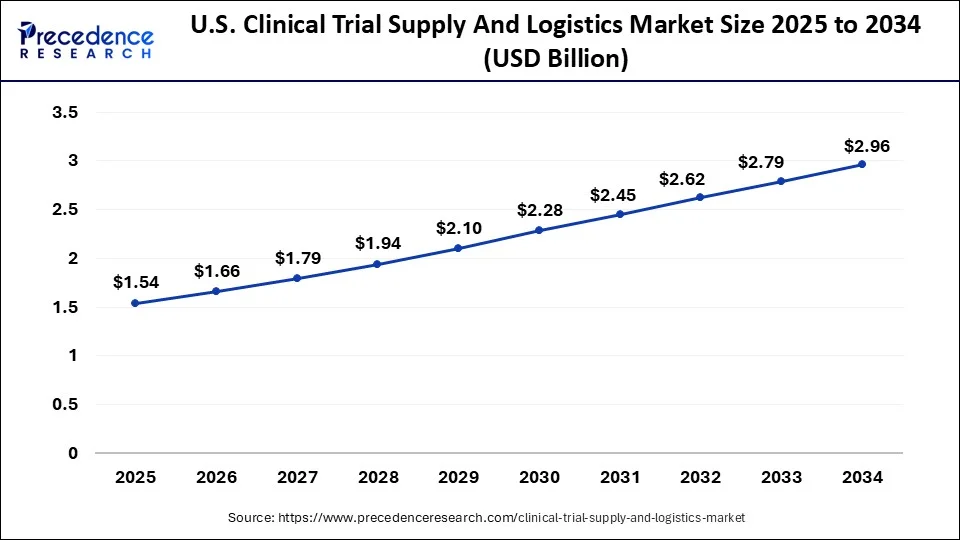
The U.S. clinical trial supply and logistics market size surpassed USD 1.44 billion in 2024 and is anticipated to reach approximately USD 2.96 billion by 2034, expanding at a CAGR of 7.53% between 2025 and 2034.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Try Before You Buy – Get the Sample Report@ https://www.precedenceresearch.com/sample/3303
How North America Dominated the Clinical Trial Supply and Logistics Market?
North America dominated the clinical trial supply and logistics market in 2024. Drug and biotechnology research and development an international epicenters around the region, especially in the U.S. It hosts some of the largest pharmaceutical firms, contract research organizations, and academic research centers that are busy conducting clinical trials in the different fields of therapy.
Moreover, the well-developed infrastructure of the region, the use of cold chain technologies, and a high level of investment into R&D contribute to the market competence. An extremely strictly controlled setting, where regulatory bodies like the FDA are imposing strict requirements, is also a contributor to reliance on specialized logistics vendors that can make sure of compliance and product integrity.
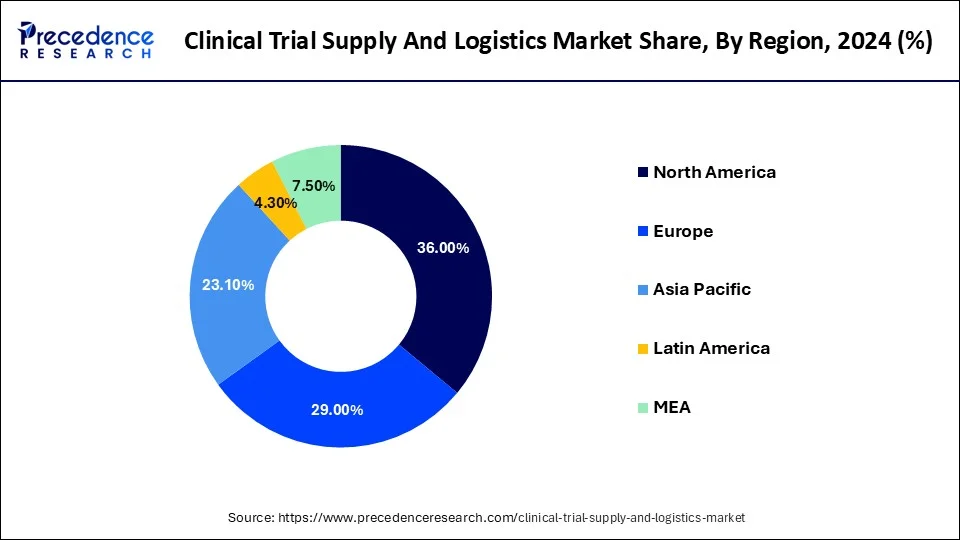
Why is Asia Pacific the Fastest-Growing in the Clinical Trial Supply and Logistics Market?
Asia Pacific experiences the fastest growth in the market during the forecast period. The high growth of the region is supported by the high and varied number of patients in the area, which serves as an excellent source for conducting clinical trials. Also, the affordability of the trial process in various countries like China, India, and South Korea is a plus to make multinational pharmaceutical companies and CROs spend heavily in the region.
There is also a rise in government support, an increase in healthcare spending, as well as a better infrastructure in clinical research in the Asia Pacific. The increasing cases of chronic and rare diseases also drive the increase in the number of trials, which contributes to the need to provide effective supply and logistics solutions.
Clinical Trial Supply and Logistics Market Segmentation Analysis
Service Analysis:
The logistics & distribution segment dominated the clinical trial supply and logistics market in 2024. Due to the sensitive nature of clinical trial materials, the logistics providers should ensure product integrity using advanced cold chain, temperature-controlled packaging, and real-time tracking technologies.
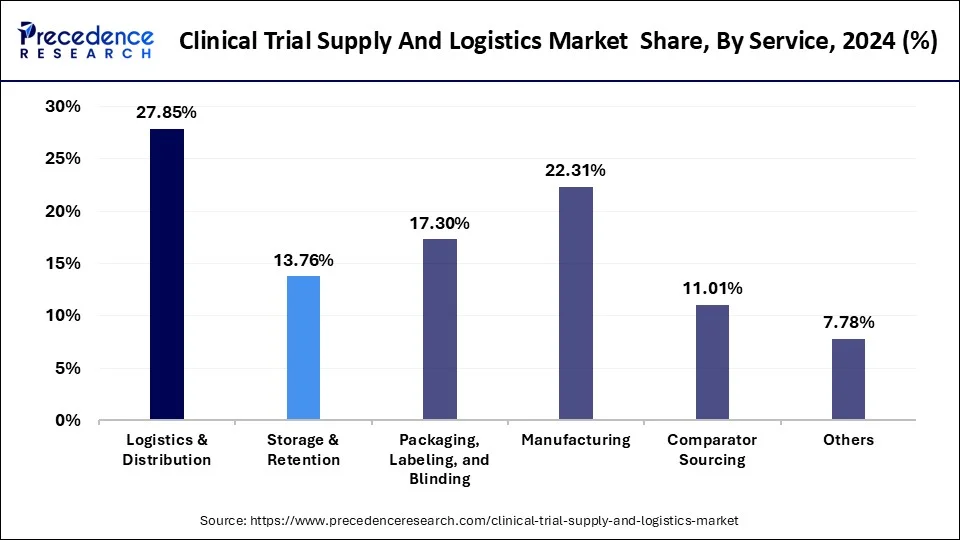
The increased complexity of global clinical trials, which in most cases involves the use of multiple countries and hundreds of sites, has further increased the need to use efficient logistics and distribution services. Also, the growing scope of this segment is due to the need for last-mile delivery solutions because of the adoption of new models of trials, decentralized and direct-to-patient.
Phase Analysis:
The phase III segment held the largest share in the clinical trial supply and logistics market in 2024. Phase III trials are commonly the largest phase of clinical research, consisting of thousands of subjects at multiple locations and often extending to more than one country. These trials require thorough logistics planning because of the scale and complexity of the trials; the supply of investigational drugs and other related materials needs to be consistent throughout all of the sites.
In contrast to the early-phase trials, phase III trials need a larger amount of drugs, a rigorous follow-up of dosing schedules, and powerful distribution monitoring systems.
End-user Analysis:
The pharmaceuticals segment dominated the clinical trial supply and logistics market in 2024. Clinical trials play an important role in the pharmaceutical industry as they are the means by which the safety, efficacy, and regulatory compliance of new drugs and therapies are established prior to being marketed.
As more pharmaceutical firms invest in their R&D and the number of pipeline drugs is increasing, they are placing more of their trial operations in specialized supply and logistics suppliers. Delivery of products to trial sites in a timely and secure manner is critical to prevent delays and to enforce patient safety.
Therapeutic Area Analysis
The cardiovascular diseases segment held the largest share in the clinical trial supply and logistics market in 2024. Heart diseases are among the most frequent causes of death on a global scale, which leads to the high demand for innovative treatments and extensive research in the field of clinical studies. This prevalence has driven an increasing number of cardiovascular clinical studies, which have necessitated robust logistics to coordinate the delivery of study drugs, equipment, and monitoring apparatus to various locations and different regions. Also, continuous progress in drug development, biologics, and medical devices to address cardiovascular health is driving trial presence within this therapeutic field.
Clinical Trial Supply and Logistics Market Top Companies
- Almac Group
- DHL
- Parexel
- Thermo Fisher Scientific (Patheon)
- Marken
- Piramal Pharma Solutions
- UDG Healthcare
- Catalent, Inc.
- FedEx
- Movianto
- Packaging Coordinators Inc.
Recent Developments
- In March 2025, DHL Group acquired CRYOPDP of Cryoport Inc. in a bid to expand its life sciences and healthcare supply chain target. CRYOPDP aims to provide solutions to clinical trial logistics, biopharmaceuticals, and cell and gene therapy, and this will assist DHL to strengthen its global presence and offer advanced, reliable, and comfortable solutions in specialty courier services.
- In November 2024, FedEx added the Korea Life Science Center in Gimpo, South Korea. To ensure that the facility meets the pharmaceutical cold chain, it will include five temperature-controlled spaces with temperatures ranging between -150 °C and 25 °C.
- In June 2024, Thermo Fisher Scientific inaugurated an ultra-cold, cGMP-compliant facility in Bleiswijk, the Netherlands. This site adds to Thermo Fisher’s strengths in contract manufacturing and specialty logistics, serving pharmaceutical and biotech companies at all stages of clinical trials.
Clinical Trial Supply and Logistics Market Segments Covered in the Report
By Service
- Logistics & Distribution
- Storage & Retention
- Packaging, Labeling, and Blinding
- Manufacturing
- Comparator Sourcing
- Others
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End-user
- Pharmaceuticals
- Biologicals
- Medical Device
By Therapeutic Area
- Oncology
- Cardiovascular Diseases
- Respiratory Diseases
- CNS and Mental Disorders
- Others
By Regions
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Thank you for reading. You can also get individual chapter-wise sections or region-wise report versions, such as North America, Europe, or Asia Pacific.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/3303
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Towards Packaging | Towards Automotive | Towards Chem and Materials | Towards FnB | Towards Consumer Goods | Statifacts | Towards EV Solutions | Towards Dental | Nova One Advisor | Market Stats Insight
Get Recent News:
https://www.precedenceresearch.com/news
For the Latest Update, Follow Us:
LinkedIn | Medium | Facebook | Twitter
✚ Related Topics You May Find Useful:
➡️ Life Science Logistics Market: See how pharma supply chains are evolving with cold chain and specialized distribution
➡️ Clinical Trials Support Services Market: Explore how CRO partnerships and outsourcing drive efficiency in trials
➡️ AI in Clinical Trials Market: Track how artificial intelligence is transforming trial design and patient monitoring
➡️ Clinical Trial Imaging Market: Understand the growing role of advanced imaging in drug development
➡️ Clinical Trial Investigative Site Network Market: Discover how site networks improve patient recruitment and trial outcomes
➡️ Clinical Trial Patient Recruitment Services Market: Analyze strategies boosting patient engagement and trial diversity
➡️ Oncology Clinical Trials Market: Examine how cancer research drives global trial investments and innovation
➡️ Neurology Clinical Trials Market: See how neurological disorder treatments are shaping trial pipelines
➡️ Clinical Data Management System Market: Explore how digital platforms enhance trial data accuracy and compliance
➡️ Virtual Clinical Trials Market: Track how remote and decentralized models reshape patient participation
➡️ Clinical Trial Management System Market: Understand how CTMS solutions streamline operations and reporting
➡️ Cell and Gene Therapy Clinical Trials Market: Discover how advanced therapies drive trial demand and regulatory focus
➡️ Clinical Trials Matching Software Market: Analyze how AI tools improve trial-patient matching efficiency
➡️ Clinical Trial Biorepository and Archiving Solutions Market: See how secure biobanking supports long-term research reliability
➡️ Cloud-Based Clinical Trial Market: Explore the adoption of SaaS and cloud platforms in clinical operations
➡️ Medical Device Clinical Trials Market: Understand regulatory and innovation trends shaping device approvals
➡️ Mental Health Clinical Trials Market: Track the growing focus on therapies for depression, anxiety, and beyond
➡️ U.S. Clinical Trial Management System Market: Analyze CTMS adoption and regulatory compliance in the U.S. market
➡️ Clinical Laboratory Services Market: See how diagnostics and lab networks support global clinical research
➡️ Cardiovascular Clinical Trials Market: Explore how cardiovascular therapies are advancing through trials
➡️ Clinical Data Analytics Solutions Market: Understand how analytics enhance trial decision-making and efficiency
➡️ Clinical Documentation Improvement Market: Discover strategies improving trial transparency and regulatory compliance
➡️ AI-Based Clinical Trials Solution Provider Market: Track how providers leverage AI to accelerate trial timelines
➡️ Clinical Trial Packaging Market: Analyze innovation in packaging design, labeling, and logistics
➡️ RNA Therapy Clinical Trials Market: See how RNA-based drugs are fueling next-generation clinical trials
➡️ Healthcare Contract Research Organization Market: Explore CRO growth driven by outsourcing in pharma and biotech
➡️ eClinical Solutions Market: Understand the shift toward digital, integrated trial solutions
➡️ Clinical Trials Site Management Organizations Market: Discover how SMOs support trial execution and patient retention
➡️ Contract Research Organization Services Market: Analyze how CRO services expand across therapeutic areas
➡️ Clinical Workflow Solutions Market: Track digital tools optimizing clinical trial workflows
➡️ Clinical Communication and Collaboration Market: See how secure communication platforms enhance trial coordination
➡️ Medical Information Market: Explore how information management supports compliance and patient safety

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

